Your cart is currently empty!
oYo-Link® Oligo Custom
Site-specific antibody-oligo conjugation in under 2 hours, with only 30s hands-on time. Label custom ss & ds oligos up to 80bp or longer.
Requirements: Photo-crosslinking Device
Site-specific antibody-oligo conjugation in under 2 hours, with only 30s hands-on time. Label custom ss & ds oligos up to 80bp or longer.
Requirements: Photo-crosslinking Device
oYo-Link® Antibody-Oligo Conjugation Reagents
oYo-Link® Oligo Custom enables the efficient and site-specific labeling of oYo-Link compatible antibodies with any oligonucleotide. Simply provide us with your oligonucleotide sequence and we send you the custom oYo-Link oligo reagent with your oligonucleotide attached. Label your antibody in just 30-seconds hands-on time.
Requirements: Photo-crosslinking Device
For Oligo lengths greater than 80bp or for purchase orders please request a quote
Note: Some oligo sequences may incur higher costs due to complex synthesis
oYo-Link® Antibody-Oligonucleotide Conjugation Reagents
oYo-Link® Oligo Custom enables researchers to label their antibodies with custom oligonucleotides for iPCR, multiplexing, cell barcoding, Proximity Ligation Assays, CITE-Seq and more. Both ssDNA and dsDNA oligos are supported, up to 80bp or longer. Fill out the checkout form to specify your oligo sequence and phosphorothioate (PS) bond modifications. The Oligo cost is included in oYo-Link pricing.
For Research Use Only. All Oligonucleotides are HPLC or PAGE grade, ensuring confidence in downstream assays.
Requirements: To label your compatible antibodies with oYo-Link®, you will need a Light Source emitting at 365nm (6-10W). Use your own Compatible Device from the list here or purchase AlphaThera’s LED Photo-Crosslinking Device Here. Oligo sequence is specified by customer at checkout.
Purchasing Information: For credit card purchases, proceed to checkout on the website. Alternatively click here to request a quote.
Technical Support: For Oligos with length > 80bp, please request a quote here. For additional questions concerning compatibility or oligo sequence specifications please get in touch.
Purchaser is solely responsible for determining whether Purchaser has all intellectual property rights that are necessary for Purchaser’s intended uses of the Product.
Webinar: Simple, fast, site-specific antibody labeling with oYo Link®
Watch our short introduction to AlphaThera’s oYo-Link® site-specific antibody conjugation technology and learn how to streamline your assays with these superior labeling products.
Antibody-Oligo Conjugation: Applications, Challenges & the Next-Gen Technology Simplifying Labeling
Antibody-oligonucleotide conjugates are becoming increasingly popular to improve assay sensitivity and specificity, and for their potential in single-cell and subcellular analysis. However the conjugation process continues to be a barrier. In this eBook we look at the key applications, the challenges and limitations of oligo conjugation, and new technologies enabling easy labeling.
Antibody Conjugation User Manual
Read our antibody conjugation usual manual for instructions on how to easily conjugate your antibody with oYo-Link products.
Demo: oYo-Link® Antibody Conjugation
Find out how to site-specifically label your antibody with just two steps: mix and illuminate. 30 seconds hands-on time, 2 hours total.
Download the oYo-Link® Oligo Custom brochure and access 20% discount off your first order
oYo-Link® Product Brochure
Download our brochure for more information and to access 20% off your first order of oYo-Link® Antibody Labeling products.
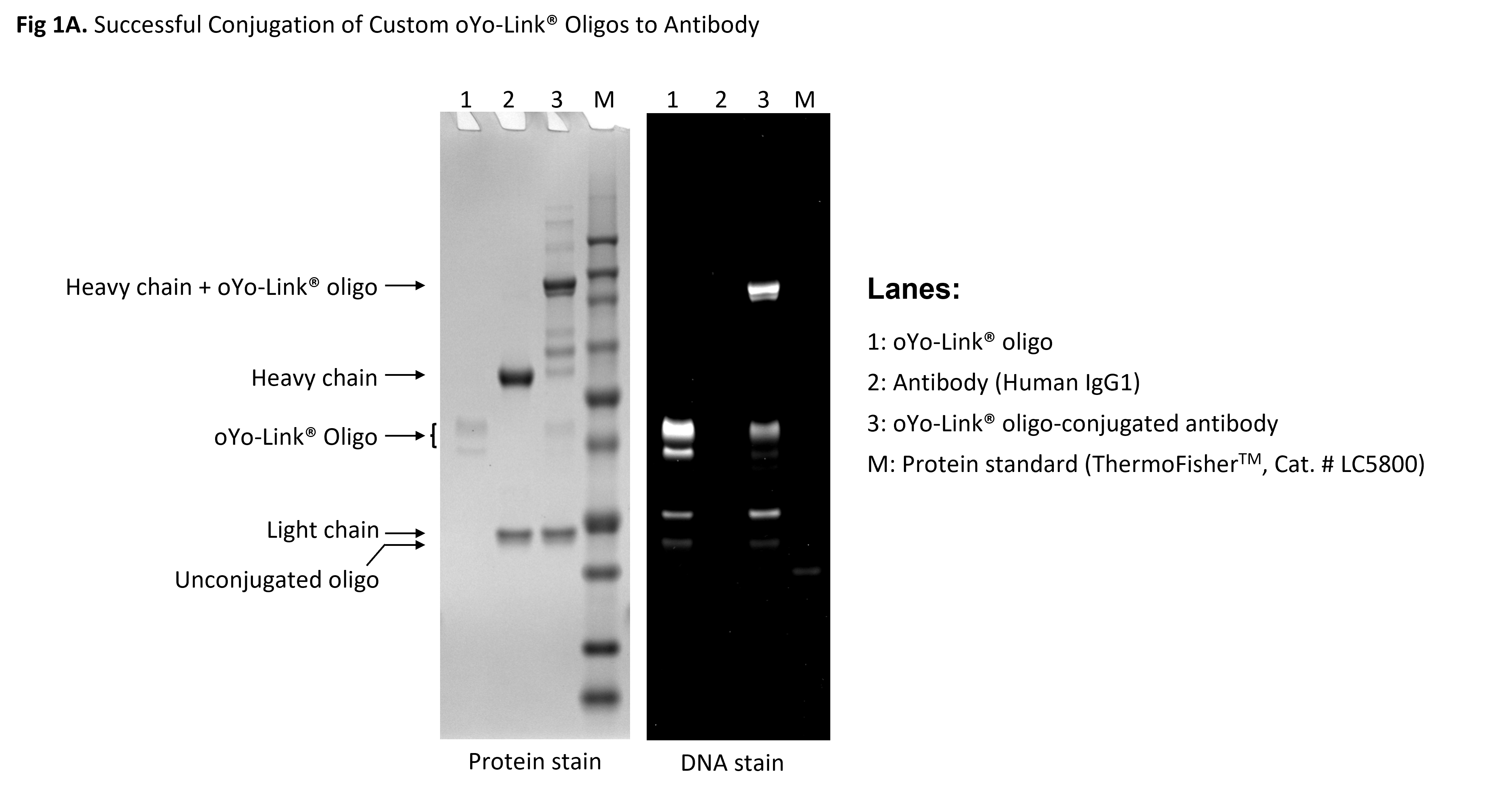
Figure 1: oYo-Link Oligo Antibody Crosslinking Data
1μL of the oYo-Link Oligo reagent was mixed with 1μg of antibody (human IgG1) and photo-crosslinked under 365nm UV for 2hrs. Samples were reduced and run on a 4-12% Bis-Tris denaturing gel. Following site-specific photo-crosslinking of the antibody‘s heavy chain with oYo-Link Oligo, an upward shift of the antibody heavy chain was observed. Fig. 1A above demonstrates over 90% conjugation efficiency of oYo-Link Oligo to the antibody.

Fig 1B. Crosslinking Data for Oligo of Varying Length & Sequence
oYo-Link allows efficient oligo-antibody conjugation using custom oligos with different sequences and length: 1μL each of the oYo-Link Oligo reagents was mixed with 1μg of antibody (human IgG1) respectively and photo-crosslinked under 365nm UV for 2hrs. Each of the five oligos has a different sequence and a different length, varying from 10nt to 80nt. Samples were reduced and run on a 4-12% Bis-Tris denaturing gel. Following site-specific photo-crosslinking of the antibody‘s heavy chain with oYo-Link Oligo, an upward shift of the antibody heavy chain was observed. The figure above demonstrates over 90% conjugation efficiency of oYo-Link Oligo to the antibody. There was no significant conjugation efficiency difference observed among oligos with different sequences and lengths.
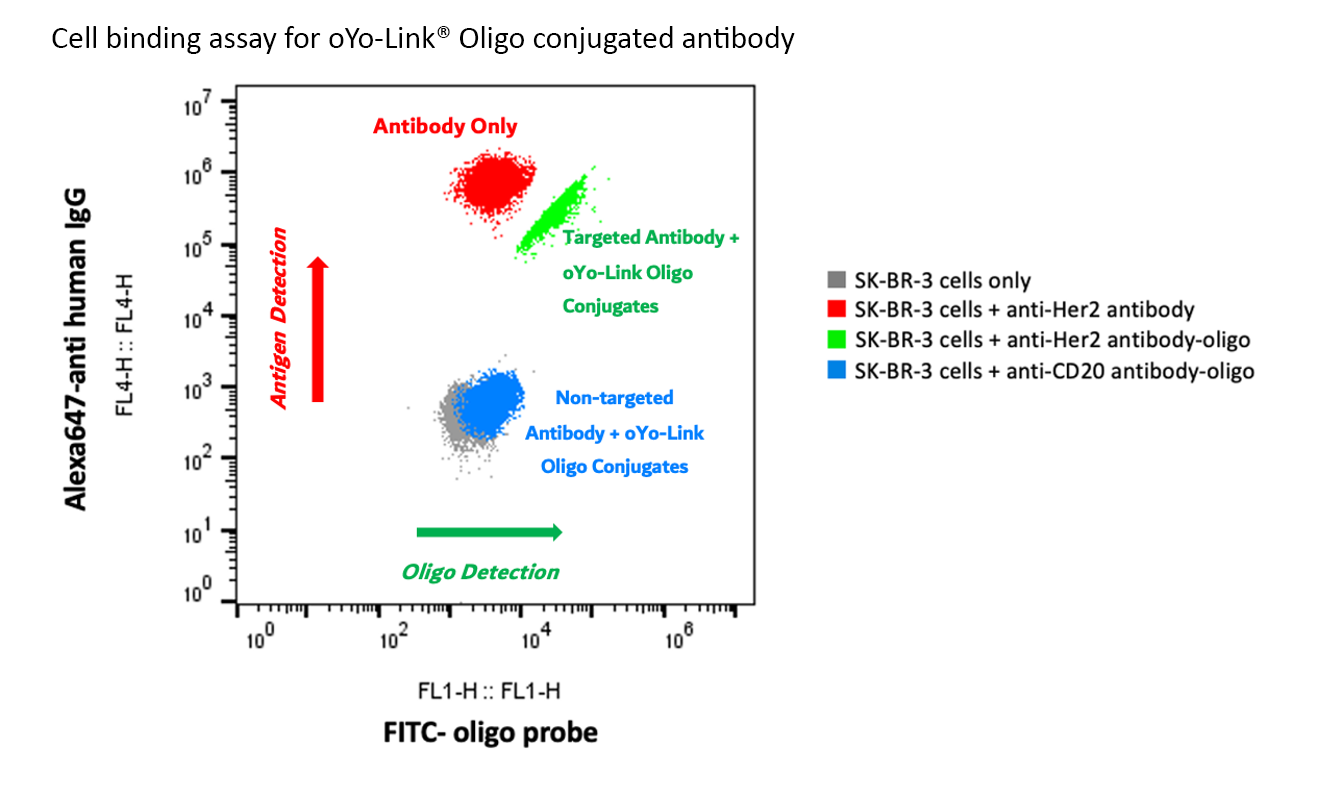
Figure 2: oYo-Link Oligo Cell Binding Assay Data
Human anti-Her2 antibody and Human anti-CD20 antibody were both conjugated with oYo-Link® Oligo via 365nm UV crosslinking. Her2 overexpressed SK-BR-3 cells were used for the cell binding assay. SK-BR-3 cells were firstly incubated with human anti-Her2 antibody with or without oligo conjugation. Human anti-CD20 antibody which also conjugated with oligo was used as a negative control. After wash, cells were further stained with an Alexa647 labeled goat anti-human secondary antibody (Invitrogen, Cat.# A21445) and a FITC labeled oligo probe for FACS analysis. The positive shift of the Alexa647 signal (FL4) indicated the anti-Her2 antibody binding to the Her2+ SK-BR-3 cells and the positive shift of the FITC signal (FL1) indicated the presence of the oligo along with the antibody binding. Cells binding with oYo-Link Oligo conjugated human anti-Her2 antibody showed strong Alexa647 (FL4) positive shift and FITC (FL1) positive shift (Green). There is no Alexa647 (FL4) signal shift observed in negative antibody control sample (human anti-CD20 antibody conjugated with oligo, Blue). There is no FITC (FL1) signal shift observed neither in no oligo conjugated anti-Her2 antibody binding samples (Red) nor negative antibody control samples (human anti-CD20 antibody conjugated with oligo, Blue). The results suggested the successful and specific binding of the oYo-Link Oligo conjugated antibody to its cellular targets.
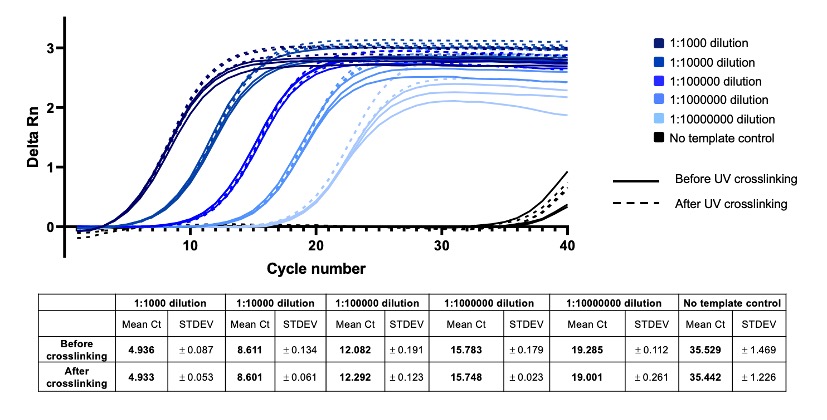
Figure 3: UV Crosslinking (365nm) does not affect PCR amplification of oYo-Link Oligo
oYo-Link Oligo before and after UV (365nm) crosslinking to anti-EGFR antibody, was used as template for Real-time PCR amplification. Starting with 16.5 µM of oYo-Link Oligo, before or after UV crosslinking, the template was diluted 1:1000 through 1:10,000,000-fold. SYBR Green was used to detect amplification products, and baseline-subtracted, normalized signal is plotted on the y-axis (Delta Rn). A no template control was also included for the Real-time qPCR assay. No significant difference is observed between cycle threshold (Ct) values for oYo-Link Oligo before and after UV crosslinking. Therefore, 365nm UV crosslinking has very minimal effects on the oYo-Link Oligo for downstream PCR-based applications.
Specifications:
| Product Name | oYo-Link® Oligo Custom |
| Catalog # | AT1002 |
| Lot # | See product spec sheet shipped with product. |
| Antibody and Buffer Compatibility | See oYo-Link Antibody & Buffer Compatibility Table here. Note: To label mouse IgG1 antibody, you must order the oYo-Link mIgG1 products, which will only label mIgG1 antibody. |
| Molecular Weight | 8000 g/mol (without Oligo) |
| Concentration | 270 µg/mL (without Oligo) |
| Molar Concentration | 33 µM |
| Storage Buffer | 1 x PBS |
| Shipping Condition | This product is shipped as a dried clear or white pellet on ice. |
| Reconstitution | Before opening, briefly centrifuge each tube to ensure that the entire pellet is at the bottom. For AT1002-25ss or AT1002-25ds Add 25 µL Nuclease-free H2O to each tube and vortex tube briefly until fully dissolved. For AT1002-100ss or AT1002-100ds Add 100 µL Nuclease-free H2O to each tube and vortex briefly until fully dissolved. |
| Storage and Stability | The dried product can be stored at 4°C or -20°C upon receipt. Following reconstitution, the product can be stored at 4°C for short-term durations (< 1 week) and -20°C to -80°C for longer time-spans. |
| Conjugation Instructions | After reconstitution, each AT1002-25 tube is capable of labeling up to 25 µg of antibody and each AT1002-100 tube up to 100 µg of antibody. Please find and follow the Antibody Conjugation User Manual here. |
| Other Notes | Disposal of product should be performed in compliance with all applicable federal, state, and local regulations. This product does not meet hazard classification criteria based on evaluations made by our company under the OSHA Hazard Communication Standard, 29 CFR 1910.1200. |

Immuno-Pcr (iPCR) is a highly powerful immunodetection method that combines ELISA specificity with the signal amplification power of PCR. Enabled by the Antibody-DNA conjugate, iPCR detection assays have shown to perform 100-1000 fold more sensitive than traditional ELISA with much less antibody used .
Read our Immuno-PCR blog Here.
Multiplexing by Antibody Barcoding or Hashtagging: Antibody-Oligo Conjugates leverage the programmability of DNA barcodes to enable high-resolution cellular and subcellular feature analysis provided by multiplexing capabilities. Hundreds of subcellular targets can be effectively labeled and imaged through the Ab-Oligo Conjugate multiplexing technique.
Proximity Ligation Assays use Antibody-Oligo conjugates to create a highly specific and sensitive immunohistochemical tool, enabled by a pair of DNA proximity probes conjugated to antibodies. This tool couples the specificity of ELISA with the powerful sensitivity of PCR to study single proteins, protein-protein interactions, post-translational modifications and more.for high-sensitivity detection of protein interactions.
Spatial profiling of human pancreatic ductal adenocarcinoma reveals molecular alterations associated with venous invasion
Bell, A.T.F., Hirose, K., Salas-Escabillas, D., Zucha, D.M., Mitchell, J.T., Danilova, L., Damanakis, A.I., Tandurella, J.A., Johnson, J., Zhu, J., Eshleman, J.R., Zhu, Q., Anders, R.A., Kagohara, L.T., Fertig, E.J., Wood, L.D. (2025). Spatial profiling of human pancreatic ductal adenocarcinoma reveals molecular alterations associated with venous invasion. Science Translational Medicine. Vol 17, Issue 817. DOI:10.1126/scitranslmed.ady752.
Cassini: Streamlined and Scalable Method for in situ profiling of RNA and Protein
Lapique, N., Kim, M.T., Thom, N., Nadaf, N.M., Pineda, J., Macosko, E.Z. (2025). Cassini: Streamlined and Scalable Method for in situ profiling of RNA and Protein. Nature Communications. https://www.nature.com/articles/s41467-025-63798-0
Deep-tissue transcriptomics and subcellular imaging at high spatial resolution
Gandin, V., Kim, J., Yang, L.Z,. Lian, Y., Kawase, T., Hu, A., Rokicki, K., Fleishman, G., Tillberg, P., Castrejon, A.A., Stringer, C., Preibisch, S., Liu, Z. J. (2025). Deep-tissue transcriptomics and subcellular imaging at high spatial resolution. Science. Vol 388, Issue 6744. DOI: 10.1126/science.adq2084
Agarose Microgel-Based In Situ Cleavable Immuno-Rolling Circle Amplification for Multiplexed Single-Molecule Quantitation on Single Extracellular Vesicles
Park, J., Feng, M., Yang, J., Shen, H., Qin, Z., Guo, W., Issadore, D.A. (2025). Agarose Microgel-Based In Situ Cleavable Immuno-Rolling Circle Amplification for Multiplexed Single-Molecule Quantitation on Single Extracellular Vesicles. ACS Nano. DOI: https://doi.org/10.1021/acsnano.5c04207
Integration of phospho-signaling and transcriptomics in single cells reveals distinct Th17 cell fates
Frey, B.F., Elahi, A., Hanumanthu, V.S., Liu, S., Goldsborough, A., Ferrell, Jr, P.B., Grant, M.B., Weaver, C.T.,
Unraveling cellular complexity with transient adapters in highly multiplexed super-resolution imaging
Schueder, F., Rivera-Molina, F., Su, M., Marin, Z., Kidd, P., Rothman, J. E., Toomre, D., Bewersdorf, J. (2024). Unraveling cellular complexity with transient adapters in highly multiplexed super-resolution imaging. Cell, [online] Mar 28;187(7):1769-1784.e18. DOI: 10.1016/j.cell.2024.02.033.
DNA-based AND logic gate as a molecular precision tool: selective recognition of protein pairs in lipid nanodiscs and subsequent binding of gold nanorods
De, S., Hechler, M., Cheng, M., Giesler, H., Brandau, S., Saccà, B., Schlücker, S. (2024). DNA-based AND logic gate as a molecular precision tool: selective recognition of protein pairs in lipid nanodiscs and subsequent binding of gold nanorods. ChemRix. DOI: 10.26434/chemrxiv-2024-dv0pj
Non-disruptive 3D profiling of combinations of epigenetic marks in single cells
, Y., , J.D.S., , M.H., , S., , Y., , Y., , M., , A.Z.,
DNA-Directed Assembly of Multivalent Lipid Nanoparticles for Targeted T Cell Gene Delivery
, M.D., , T.Q., , A.U., , Y., , A.P., , L., , C.,
Fixation Before Dissociation Using a Deep Eutectic Solvent Preserves In Vivo States and Phospho-Signaling in Single-Cell Sequencing
, S.D., , B.F., , V.S., , S., , A., , K.V., , C.T., , M.B.,
A method for Boolean analysis of protein interactions at a molecular level
Raykova, D., Kermpatsou, D., Malmqvist, T., Harrison, P.J., Sander, M.R., Stiller, C., Heldin, J., Leino, M., Ricardo, S., Klemm, A., David, L., Spjuth, O., Vemuri, K., Dimberg, A., Sundqvist, A., Norlin, M., Klaesson, A., Kampf, C. and Söderberg, O. (2022). A method for Boolean analysis of protein interactions at a molecular level. Nature Communications, [online] 13(1), p.4755. DOI:https://doi.org/10.1038/s41467-022-32395-w.
Efficient DNA fluorescence labeling via base excision trapping.
Jun, Y.W., Harcourt, E.M., Xiao, L., Wilson, D.L. and Kool, E.T. (2022). Efficient DNA fluorescence labeling via base excision trapping. Nature Communications, [online] 13(1), p.5043. DOI: https://doi.org/10.1038/s41467-022-32494-8.
Does the oYo-Link Oligo price include oligo synthesis cost?
Yes, the cost of the oligonucleotide is included in our price. Please fill in the form with your oligo sequence. All oligos are synthesized by Integrated DNA Technologies. The oligos are HPLC or PAGE grade.
Does 2 hours of UV illumination damage the oligo?
No, the antibody-oYo-Link Oligo conjugation is performed under 365nm wavelength which is very safe for the oligo. No degradation or mutation has been found.
What is the length of oYo-Link Oligo that AlphaThera can provide?
Our standard pricing applies to oYo-Link Oligos up to a length of 80 nt. The antibody conjugation efficiency is >90% for oligos of all lengths. Information can be found here. Additional information can be found on the Oligo product page here. oYo-Link Oligos longer than 80 nt can also be made, but need to be special ordered. Please contact support@alphathera.com
How long will it take to receive my oYo-Link Oligo once I place the order?
It usually takes 1-2 weeks from the time of processing the order until shipping the product out.
Are oYo-Link Oligos attached to the antibody at the 5’or 3’ end?
oYo-Link Oligos can be attached to antibodies at either the 5’ or 3’ end. You can specify your preference at the time of ordering. The conjugation efficiency is the same.
Can I have oYo-Link Oligo prepared with a fluorescent label?
Yes. If the oligo 5’ end is attached to oYo-Link, then the fluorescent dye can be attached at the 3’ end or vice versa. This will be a special order. Please contact customer support at support@alphathera.com.
How stable is oYo-Link Oligo?
oYo-Link Oligo is very stable. Heating up to 75°C for 5 mins and cooling down will not affect the antibody conjugation efficiency.
How should I store the conjugated Antibody-oYo-Link Oligo?
Please store the antibody-oYo-Link Oligo conjugate according to the antibody vendor’s recommendation.
Can oYo-Link Oligo be cleaved from the antibody following conjugation?
One strategy to cleave oYo-Link Oligo from the antibody is to insert a restriction enzyme sequence into the oligo sequence. Please note that most digestion enzymes work for dsDNA. oYo-Link Oligo is not UV cleavable.
Can I order oYo-Link Oligo with an RNA sequence?
Since RNA oligos are prone to degradation and unstable, we are not currently offering this option; however, oYo-Link Oligos can be made with U’s in the oligo sequence, but it will be on a DNA backbone
Will oYo-Link work with my antibody?
Please check the antibody compatibility table. Note: oYo-Link mIgG1 can be used to label mouse IgG1 antibody. Currently, oYo-Link mIgG1 is only available for certain products. Please check the product webpage. oYo-Link mIgG1 only labels mouse IgG1 and is not compatible with other antibodies.
Can oYo-Link be used to label antibodies in the presence of albumin and/or Tris?
Yes, since oYo-Link specifically bind to the heavy chain of IgG, it works in the presence of both albumin and Tris. oYo-Link is compatible with all common buffers. See the full buffer compatibility table here.
If an oYo-Link product has accidentally been left at room temperature for a week, will it still work?
With the exception of oYo-Link DBCO, all oYo-Link products are shipped as white or clear pellets and are very stable. While we recommend cold storage, they will not exhibit any loss of activity if left at room temperature for a week. oYo-Link DBCO must be shipped and stored cold (-20°C) immediately upon arrival, otherwise there will be a loss in activity.
Does AlphaThera offer conjugation service?
Yes. If you are interested in our conjugation service, please contact us at support@alphathera.com. For custom conjugation services, you will need to send us your antibody. The conjugation and purification fee depends on the size and specifications of each order. Purification is not required for most applications.
How many oYo-Link labels will be conjugated to each antibody?
For most subclasses and species of antibody, oYo-Link will result in the conjugation of 2 labels per antibody (maximum), one label per heavy chain. For example, ~95% of human IgG and Rabbit IgG will have two labels per antibody. However, there are a few antibodies such as mouse IgG2b and goat IgG, where the conjugation is slightly less efficient. In this case, 60 to 80% of the antibody will be labeled and have a mixture of 0, 1 and 2 labels per antibody. See the antibody conjugation efficiency here
How do I know if oYo-Link was successfully conjugated to my antibody?
Antibody conjugation can be checked on SDS-PAGE gel. An example is present in the supporting data on each product page.
Does 365nm UV illumination damage the antibody and its binding affinity?
The conjugated and unconjugated antibody binding affinities have been tested via both ELISA and cell binding assays and don’t show any difference.
Do I need to remove free oYo-Link after conjugating to antibody?
For most immunoassay applications, purification is not necessary. In our standard recommended protocol, the molar ratio of oYo-Link-to-antibody is 5:1. These conditions ensure that the antibody conjugation efficiency reaches its maximum; however there will be a slight excess remaining in your antibody mixture. This is usually removed during immunoassay washing steps. However, if your application requires high antibody-conjugate purity, please contact us for more information on this process and for a detailed protocol.
For oYo-Link click-chemistry products, should I perform the click reaction before conjugation?
When working with oYo-Link Azide (Catalog #: AT3002) and oYo-Link DBCO (Catalog #: AT3003), it is required to perform the click chemistry coupling reactions with the molecule of interest first, prior to photo-crosslinking to an antibody. This is to avoid UV illumination damage to the click moiety and to avoid diluting oYo-Link prior to the reaction to maximize the reaction efficiency. For oYo-Link Tetrazine (Catalog #: AT3004) and oYo-Link Thiol (Catalog #: AT3001); it is recommended to perform the coupling reaction before photo-crosslinking. This is to avoid diluting oYo-Link prior to the coupling reaction to maximize the reaction efficiency.
What is the lowest amount of antibody that can be labeled by oYo-Link reagents?
We recommend working with at least 1 µg of antibody; however, it is possible that even lower amounts of antibody can be labeled.
Can antibodies that are highly dilute be labeled by oYo-Link reagents?
Yes, antibodies can be efficiently labeled even if diluted to concentrations as low as 50 µg/mL.
oYo-Link® Oligo Custom Advantages
- Label ss & ds Oligos <80bp or longer
- Rapid Labeling
<30 seconds “hands-on” time, 2 hours total. Only 2 steps, mix and illuminate. No need to optimize reaction conditions. - Label Small Amounts
Label as little as 1 µg of Antibody per reaction. - Label Diluted Antibody Solutions
Even those below a concentration of 50 µg/mL. - Site-Specific
Site-specific & covalent labeling of antibodies in the Fc region, results in improved antibody performance, uniform product, and consistent, predictable results. - Compatible with all common buffers
Even those that contain storage proteins and amines. - Efficient & Compatible with Antibodies
>90% labeling of most antibodies. - Save for Later
After resuspension, oYo-Link® products can be aliquoted and saved for future reactions.
Please Select Epitope Tag Options
4 EPITOPE TAGS: Please select only 4 Tags Below:
Each tag will be able to label the amount of Ab you selected on the previous page.